CHARACTERIZATION TOOLS FOR FUNCTIONAL MATERIALS
Deux méthodes de préparation des verres de chalcogénures sont utilisée au laboratoire: (i) synthèse en tube silice sous vide dans un four et (ii) synthèse par broyage mécanique à température ambiante.
Two methods for the preparation of chalcogenide glasses are used in the laboratory: (i) synthesis in a silica tube under vacuum in an oven and (ii) synthesis by mechanical grinding at room temperature.
For the first synthetic technique, the starting materials are purified and placed in a silica tube under vacuum (Figure 1). The tubes are heated in an oven to melt and homogenize the mixture. The Laboratory has 3 well-type tube furnaces, a tilting furnace and two electric furnaces with an operating temperature of 20 to 1200°C (Figure 2). Then quenching in air or in cold water is carried out to obtain a glass (Figure 3).
 |  |
| Figure 1. Vacuum assembly (approx. 10-6 mbar) used for the synthesis of chalcogenide glasses. | Figure 2. Furnaces used for the synthesis of chalcogenide glasses. |
 |  |
| Figure 3. Examples of chalcogenide glasses obtained by the method of quenching in a silica tube under vacuum. | |
A second method of synthesis is to use mechanical energy instead of thermal energy to cause a chemical reaction. For this, the grinding of the raw material is carried out in a ball mill (Figure 3) by ball impacts against the walls of the tank and between the balls themselves to obtain an amorphous and homogeneous micrometric powder.
 |
| Figure 3. Planetary Micro Mill Pulverisette 7 premium line. |
The hygroscopic nature of some glasses and starting materials requires their development and characterization in an inert environment. The glove box (Figure 4) is used to avoid the oxidation of glasses during their synthesis and characterization.
 | Caracteristics :
|
| Figure 4. Glove box “Plas-Labs Nitrogen Dry-Box Glove Box”. | |
The study of electrical transport in glasses is done using a combination of different analysis techniques (Figure 1) such as complex impedance spectroscopy for glasses with high conductivity, resistivity measurements for insulating samples (Figure 2) and the Wagner method as well as the radioactive tracer diffusion method performed at the University of St. Petersburg. First of all, the complex impedance spectroscopy/resistivity meter allows us to have information on the electrical properties of materials such as the total conductivity. Then, we can use the Wagner method which allows us to measure the electronic conductivity of materials. Diffusion by radioactive tracer is a very powerful experimental technique for studying ion transport phenomena in solids. The combination between these three techniques gives us the possibility to unambiguously determine the ionic and electronic contributions of the conductivity.
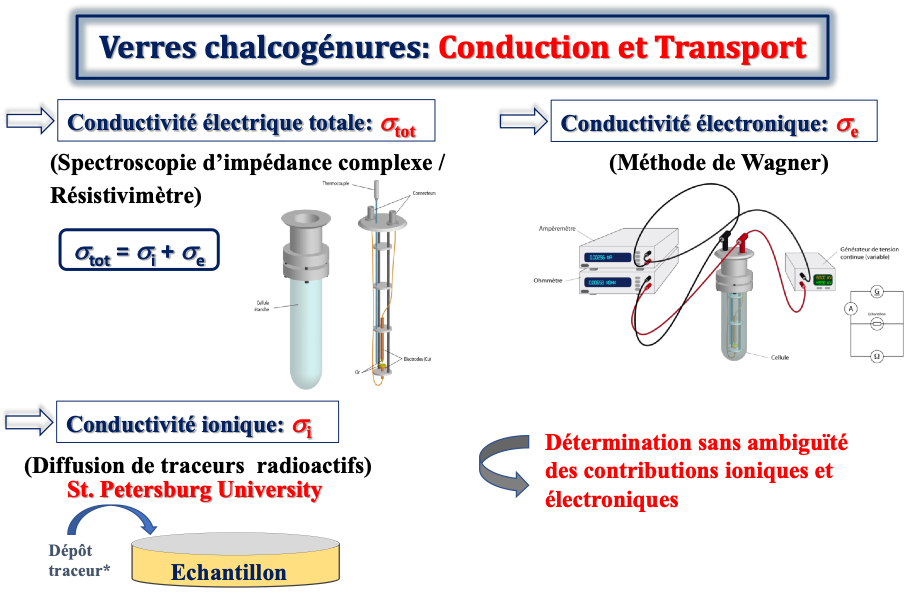
Figure 1. Analytical techniques used to study electrical transport in glasses.
 | RESISTIVIMETER IMPEDANCE-METER |
| Figure 2. Experimental device for measuring the conductivity of solids | |
The thermal properties of the glasses are analyzed using a TA Instruments Q200 Differential Scanning Calorimeter (DSC) (Figure 1). Two cooling systems are available:
- The Liquid Nitrogen Cooling System (LNCS) offering a programmable cooling function from 550 to -180°C.
- Finned Air Cooling System (FACS) which uses ambient air to cool the DSC cell and provides stable baselines and linear heating and cooling rates between ambient temperature and 725°C.
 |
Figure 1. TA Instruments DSC Q200 differential scanning calorimeter |
The Laboratory has extensive experience in the manufacture and characterization of chemical sensors with a chalcogenide glass membrane for the detection of metal cations (Figure 1). The calibration of the sensors makes it possible to define the sensitivity, the detection limit (Figure 2), the selectivity coefficients in the presence of interfering ions, the reproducibility and the influence of pH. The experimental device for calibrating sensors with an assembly of Mettler-Toledo titrators is shown in Figure 3.
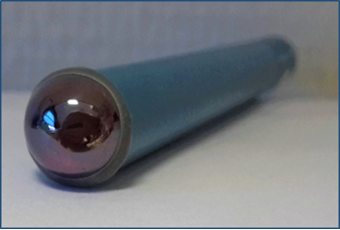 | 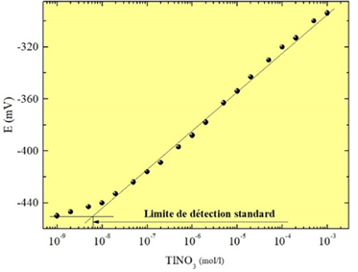 |
| Figure 1. Chemical sensor with a chalcogenide glass membrane | Figure 2. Calibration of sensor sensitive to thallium ions |
 |
| Figure 3. Experimental device with Mettler-Toledo DL7x Titrators for the calibration of chemical sensors. |


